|
Volume 18, No. 1 - Winter 2010 |
Subscribe to AT Messenger Download PDF Viewer |
| PDF Version (for printing) Large Print (PDF) Text Version |
AstraZeneca Donation Makes Communication Devices Available to Delawareans
When speaking is a challenge that seems insurmountable, then it is important to find an alternative means of communication. Enter communication devices of all shapes, sizes, and capabilities. Communication devices, also known as augmentative and alternative communication (AAC) devices, come with a seemingly endless range of functions. They can be low-tech, like the Picture Exchange Communication System (PECS), which uses simple pictures for expressive purposes. High-tech options abound and seem to increase exponentially with expanded capabilities every time you turn around. Then, of course, there are mid-tech communication devices. DATI recently received a sizeable donation of mid-tech devices from AstraZeneca, a local company, and we are making them available to Delawareans with disabilities.
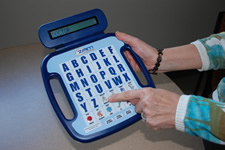 AstraZeneca, a Delaware-based pharmaceutical company, developed the ZAM Communicator. This simple communication device was created to positively impact health and communication between patients and medical professionals. The ZAM Communicator is two-sided, lightweight, and easily transportable. It enables a person to communicate by spelling out words using a keypad or pressing icons to display a message on a small LCD screen.
AstraZeneca, a Delaware-based pharmaceutical company, developed the ZAM Communicator. This simple communication device was created to positively impact health and communication between patients and medical professionals. The ZAM Communicator is two-sided, lightweight, and easily transportable. It enables a person to communicate by spelling out words using a keypad or pressing icons to display a message on a small LCD screen.
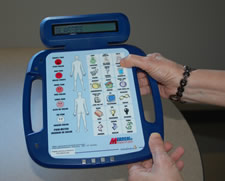 The ZAM Communicator does not have voice output but offers a double-sided display screen so that someone standing in front of the person using the device can see the text display. This device has a keypad of letters, arranged alphabetically, on one side, along with ten pre-programmed picture buttons—doctor, nurse, hot, cold. Pre-made icons fill the other side of the device and include a simple pain scale along with eighteen buttons that express feelings, needs, and wants—sick, dizzy, thirsty, hungry, glasses, read. All of the picture buttons are labeled in both English and Spanish. The ZAM is rechargeable, but the battery cannot be changed. These devices are available, free of charge, on a first-come, first-served basis to Delawareans with disabilities.
The ZAM Communicator does not have voice output but offers a double-sided display screen so that someone standing in front of the person using the device can see the text display. This device has a keypad of letters, arranged alphabetically, on one side, along with ten pre-programmed picture buttons—doctor, nurse, hot, cold. Pre-made icons fill the other side of the device and include a simple pain scale along with eighteen buttons that express feelings, needs, and wants—sick, dizzy, thirsty, hungry, glasses, read. All of the picture buttons are labeled in both English and Spanish. The ZAM is rechargeable, but the battery cannot be changed. These devices are available, free of charge, on a first-come, first-served basis to Delawareans with disabilities.
Free is, of course, a great deal, but it is important to know that there are restrictions with regards to who can obtain the ZAM. The devices may only be distributed to people with communication limitations. Health care and disability professionals are asked to promote the availability of these devices and direct individuals to contact the Assistive Technology Resource Center (ATRC) in their county. To put it plainly, we cannot distribute these communication devices to professionals. Secondly, even though the ZAM Communicator is free, it is not for everyone. Remember, it is a simple device without audio output, and it cannot be programmed. With that said, the ATRC nearest you has a wide range of augmentative and alternative communication (AAC) devices available for you to try out. These devices range from low-tech communication boards with interchangeable pictures to high-tech speech generating devices.
If you or someone you know could use a ZAM Communicator, please call, email, write, or send a carrier pigeon to your local ATRC to let them know that you would like to see if the ZAM is the device for you. The AT Specialist will set up a time for you to come in, see how it works, and, if you decide it is a good fit, then you will walk out with a ZAM. During your appointment, you will also have the opportunity to try out other communication systems and borrow one or more of them at that time. We invite you to call DATI at 1-800-870-DATI (3284) or you may visit www.dati.org and click on the “contact us” button.
Back to topDATI Receives Grant for Educational AT
More Inventory Additions for Education
Fair Housing Discrimination Against People with Disabilities
Communication Devices Available

